Background: Mantle cell lymphoma (MCL) is a moderately aggressive and incurable small to medium sized B cell lymphoma. No standardized treatment exists for this disease. Cladribine is a hypomethylating agent that indirectly down-regulate DNA methylation to suppress tumorigenesis. Combination of Cladribine and Rituximab showed a synergetic effect in treating B cell lymphomas. Bortezomib (Velcade) is an FDA approved proteasome inhibitor to treat relapsed/refractory MCL. In this study, we evaluated the safety and efficacy of Valcade, Cladribine, and Rituximab (VCR) combination treatment in newly diagnosed and relapsed/refractory MCL.
Patients and Methods: This is a single arm open label phase I trial. This study employed a standard 3+3 dose escalation scheme designed to determine the maximum tolerated dose (MTD) of Cladribine in VCR regimen. Briefly, the treatment scheme was consisted of 6 28-day cycles. At first cycle of the treatment, Rituximab 375 mg/m 2 infusion started on day 5 of the first week and then given weekly for 3 weeks; in the next 5 cycles Rituximab was given on day 5 of each cycle, and then every 2 months as the maintenance therapy. Cladribine 3 to 5 mg/m 2 (3 mg/m 2 for level 1, 4 mg/m 2 for level 2, and 5 mg/m 2 for level 3) infusion was given on days 1 to 5 for 6 cycles. For patient older than 70 years old, Cladribine was only given on days 1 to 3 of each cycle. Velcade 1.6 mg/m 2 subcutaneous injection was given on days 12, 19 and 26 for cycles 1 to 3, Days 5 and 19 for Cycles 4 to 6, then once per month as maintenance therapy. The primary endpoint of this study was the dose limiting toxicity (DLT), safety, and efficacy of this regimen in patients with MCL. The secondary endpoints included remission rate (RR), progression free survival (PFS) and overall survival (OS) (Figure 1). The analysis was done by intent to treat. Differentially methylated region (DMR) analysis and cytokine assay were performed to explore biomarkers differentiating good-responders from poor-responders.
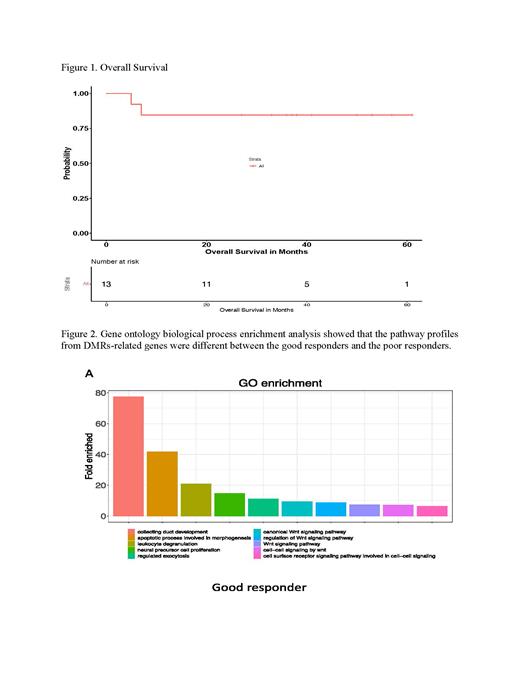
Results: This study recruited 13 patients, with a median age of 64 years old (range from 55 to 83) and including 11 males and 2 females. Ten patients were never previously treated, other 3 either relapsed or failed from previous treatments. Most patients tolerated this regimen well. No patient experienced DLT at either level 1 or 2, one possible DLT (infectious colitis) was observed during 2nd cycle at level 3. Two relapsed patients experienced disease progression after received two cycles of treatment and died in 5 and 7 months. Another patient died in 15 months after receiving treatment. The overall remission (OR) rate was 85% (11/13) and complete remission (CR) rate was 77% (10/13). In newly diagnosed patient cohort, CR rate was 100% (10/10), OS rates were 90% (9/10), and PFS was 80% (8/10), of which 3 patients have been followed for more than 4 years, 3 other patients for more than 3 years, and 3 others for over 2 years (Fig.1). In relapsed/refractory patient cohort, 1 achieved CR for 7 months but then relapsed, 2 had no response. There were 2 blastoid patients, 1 of these 2 patients was newly diagnosed and achieved CR.
Bone marrow suppression caused prolonged anemia and thrombocytopenia were the most common toxicity (3/13 with ≤grade 2). Low grade peripheral neuropathies (≤grade 2) were observed in 15% (2/13). Mild fatigue was a frequent complaint. Cytokine assay showed decreased CXCL12 level in the good responders. Though global methylation pattern showed no difference, DMRs were identified in the good responders and the poor responders. DMRs-related genes involved in CXCL2 pathways were further extracted and enriched biological process analysis revealed the pathway profile difference between good responders and poor responders. Good responders exhibited alternation of apoptotic and Wnt signaling pathways (Fig.2).
Conclusions: The VCR combination is a well-tolerated, low toxicity and effective regimen for MCL, especially for untreated MCL. Cladribine 5 mg/m 2 is a tolerable dose with manageable toxicity. Individual patient methylation profile may affect response to VCR therapy.
Disclosures
Loughran:Kymera Therapeutics: Consultancy, Current holder of stock options in a privately-held company; Recludix Pharma: Consultancy, Current holder of stock options in a privately-held company; Keystone Nano: Consultancy, Current holder of stock options in a privately-held company; Flagship Labs 86: Consultancy; Dren Bio: Consultancy, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal